It’s World Oceans Day, a day to celebrate and honour our planet’s oceans, as well as to educate the public on the ways that we are impacting it. The ocean covers most of the surface of our Earth (over 70% of it) and holds the vast majority of our water (over 96%)1 – the life force of our planet. It houses most of our planet’s biodiversity, provides us with over 50% of our oxygen, and supports the livelihoods of over 40 million people2. Needless to say, we need our oceans, yet we are on track to irreparably damage them through the process of climate change.
I have always had a fascination, and perhaps a healthy fear, for our oceans. In an attempt to tackle that fear, and to satisfy my ever-adventurous spirit, I completed an Open Water PADI diving course while on a trip to Costa Rica last fall. During the course, I spent two days diving near Isla de Caño (or Caño Island), a small, protected island off the west coast of Costa Rica. It’s a biological reserve that is only accessible if you have a reservation, and thus the ocean’s creatures in this area are relatively undisturbed. I had watched many documentaries of our underwater world, but it was something else to see it in person. I saw multiple blacktip reef sharks (Carcharhinus melanopterus), large green sea turtles (Chelonia mydas), various rays, massive schools of fish, and the most colourful corals and underwater vegetation. It was like I was on an entirely different planet.






Diving in Costa Rica in December 2022. Photographs by Firmiano Filho.
But it wasn’t a different planet. It is ours, and it’s the only one we have. Every boat ride home at the end of the day, I fought back tears of frustration at the way that humans are negatively affecting this pale blue dot3. We have all heard of pollution or micro-plastics affecting our oceans, but I have found that the topic of ocean acidification is one that many people have never even heard of. Yet, it is impacting our oceans the world over, at such a massive scale that we are at risk of losing the entire oceanic food web. In my opinion, this illustrates another way that the media has neglected to adequately describe the extent and urgency of the climate crisis.

Pale blue dot. Photograph by NASA, 1990. See more information here.
Let’s start from the beginning. You can read how climate change works in my previous blog post here. I explain how rising greenhouse gas emissions is causing Earth’s temperature to increase. But what is often misunderstood is where that heat is actually going. Naturally, the ocean absorbs more heat than the surface of the Earth does4 – this makes sense as it covers more area. Evaporation and ocean currents help to distribute this heat around the globe4. However, with increasing temperatures caused by climate change, the ocean has been absorbing more and more heat over time. In fact, over 90% of the increase in temperatures since climate change began have been absorbed by the oceans alone5. This increase in warmer waters has caused sea level rise, coral bleaching, more intense hurricanes5, as well as thermal displacement, or the shifting of marine species’ habitats6, and more.
But heat isn’t the only thing that oceans are absorbing – they are also absorbing the primary greenhouse gas causing that heat increase, carbon dioxide (CO2). About 30% of CO2 that is released into the atmosphere is absorbed by the oceans, so when the total amount of it increases, so does the amount that the oceans absorb7. Briefly, when CO2 and seawater combine, a chemical reaction occurs that causes the water itself to become more acidic – in other words, CO2 lowers the pH of seawater7-8. At first, scientists didn’t realize this was a problem. It is natural for the ocean to absorb CO2 as it already exists in our atmosphere – the ocean has a natural buffering effect that keeps the pH stable when CO2 is absorbed8. However, because the amount of CO2 in the atmosphere has been increasing since the beginning of the industrial revolution, there is now more in the ocean than it can naturally buffer8. It has been estimated that the ocean has absorbed more than 500 billion tons of CO2 from the atmosphere in that time8.
The chemical process works like this: when CO2 and water combine, they form carbonic acid7. This acid in and of itself is weak, but it releases hydrogen ions, which combine with other molecules and causes the pH to decrease, causing an increase in acidity7. One of the other molecules that hydrogen ions bond with is carbonate, making it less available to the surrounding water7-8. Carbonate is used by any creature in the ocean that creates a shell, from zooplankton, to corals, to oysters and mussels8-9 – they combine it with calcium to create calcium carbonate, which is what their shells are made of10. When carbonate is bound up with hydrogen, these critters cannot access it8, meaning that they cannot build their shells as effectively as they could before our oceans started becoming more acidic8-9. This is causing shellfish to grow to smaller sizes or to create weaker shells11. Shells are absolutely vital to the survival of shellfish – they protect their soft inner organs – so when these shells are weaker, they either simply don’t grow to maturity, or they are more at risk from predation11.
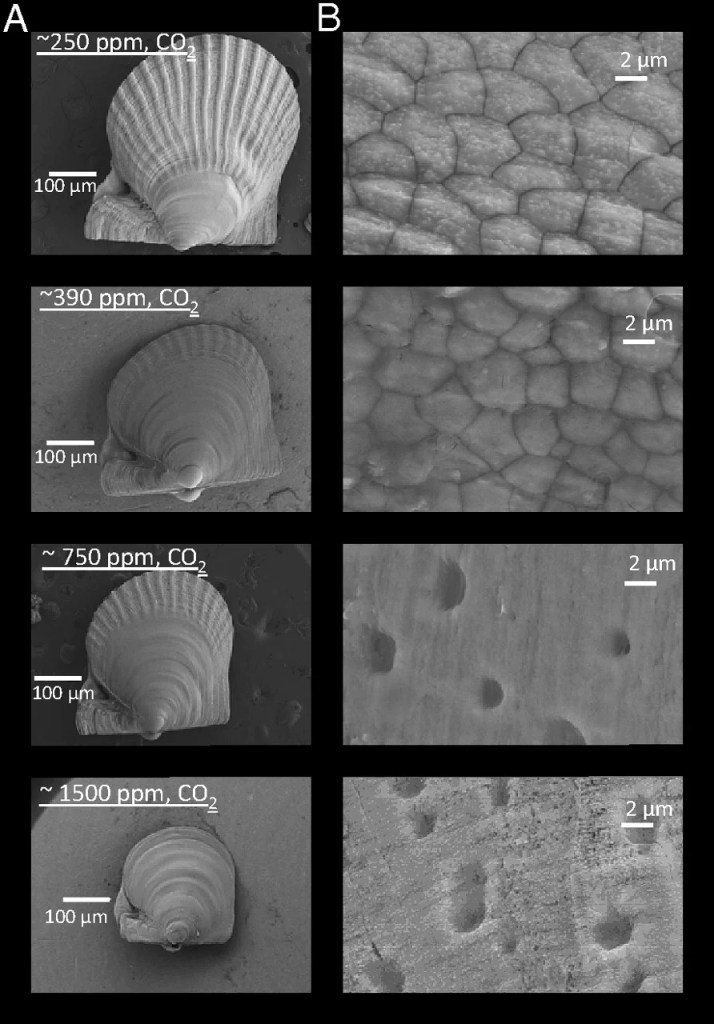
This image illustrates how different CO2 levels impact A) the size of a full-size shellfish at its larval stage and B) the structure of it’s outer shell (11). CO2 levels increase from top to bottom in the image, with the bottom illustrating the highest concentration of CO2 with the smallest and less in-tact shell (11).
There are other ways that ocean acidification is causing issues in marine ecosystems8, but this alone should make us stop procrastinating the mitigation of climate change. Many of the creatures that require calcium carbonate for their survival are the basis of the marine ecosystem – these animals are the food source for everything else within this system. Without them, we risk losing everything else that calls the ocean home. If the ocean houses most of our planet’s biodiversity, how foolish would it be for humanity to continue harming it?
As always, don’t just trust my word. Below are resources where you can learn more. Most of the sources I have included are not peer-reviewed literature. Instead, I have curated an assortment of publicly-available reliable sources from some of the world’s leading scientific organizations. Not all important information is hidden in university libraries – these organizations work hard to get this information out there for the public’s best interest. Please read it for yourself.
Sources
- United States Geological Survey. (2022). Top 10 things you didn’t know about the ocean.
- United Nations. (2023). Planet Ocean: tides are changing.
- Cornell Chronicle. (2020). Iconic ‘pale blue dot’ photo – Carl Sagan’s idea – turns 30.
- National Oceanic and Atmospheric Administration: Ocean Exploration. (n.d.). How does the ocean affect climate and weather on land?
- NASA Global Climate Change. (2022). Ocean warming.
- National Oceanic and Atmospheric Administration: Fisheries. (2020). Ocean heatwaves dramatically shift habitats.
- National Oceanic and Atmospheric Administration: National Ocean Service. (n.d.). What is ocean acidification?
- Smithsonian. (n.d.). Ocean acidification.
- National Oceanic and Atmospheric Administration: The Integrated Ocean Observing System. (n.d.). Ocean acidification.
- United States Environmental Protection Agency. (2022). Effects of ocean and coastal acidification on marine life.
- Talmage, S. C. & Gobler, C. J. (2010). Effects of past, present, and future ocean carbon dioxide concentrations on the growth and survival of larval shellfish. PNAS, 107(40): 17246-17251.
